Nature of Electromagnetic Radiation
Nature of Electromagnetic Radiation: Overview
This topic covers concepts, such as, Electromagnetic Radiations, Wave Nature of Electromagnetic Radiations, Frequency of Electromagnetic Radiations and Wavelength of Electromagnetic Radiations etc.
Important Questions on Nature of Electromagnetic Radiation
We can say that the energy of frequency is given by , where is Planck's constant. The momentum of a photon is where is the wavelength of photon. Then we may conclude that velocity of light is equal to:
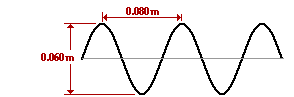
What is the amplitude of the electromagnetic wave in the diagram below?
The number of wavelengths per unit length is called
What is/are correct about Hertz ?
waves of an electromagnetic radiation passes in minutes through a point then what will be the wavelength of radiation?
The ratio of energy to the frequency of electromagnetic radiation is called:
Calculate the wavenumber of yellow radiation having wavelength ?
What will be the wavelength of the electromagnetic waves transmitted from the source, if a source is transmitting electromagnetic wave of frequency Hz ?
An object emits two different radiation, when it absorbs certain amount of energy having of wavelength in which one radiation having What is the wavelength of other radiation?
Which of the following is true for an electromagnetic radiation
Which value is closest to the wavelength, in meters, for a quantum of light with a frequency of per second?
[Given: ]
Which of the following is correct?
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of 2ns and the number of photons emitted during the pulse source is 2.5 x 1015, If the energy of the source is Y x 10-10 J. Then find the value of Y. (Report your answer in two decimals)
Emission transitions in the Paschen series, end at orbit n = 3 and start from orbit n and can be represented as Calculate the value of if the transition is observed at .
Wavenumber for a radiation having 5800 Å
wavelength is x × 10 cm–1 . The value of x is
_____.
A hypothetical electromagnetic wave is show below.

The frequency of the wave is x × 1019 Hz.x = ______ (nearest integer)
The candela is the luminous intensity, in a given
direction, of a source that emits monochromatic
radiation of frequency 'A' × 1012 hertz and that has
a radiant intensity in that direction of
watt per steradian. 'A' and 'B' are respectively
Given wavelength of wave is . If its wave number is . The value of is (Nearest integer)
Arrange in increasing wave length order:
Gamma rays, X-rays, UV rays, IR rays
The frequency of an electromagnetic radiation is What is its wavelength in metres?
(Velocity of light )
